Abstract
Introduction: Autologous stem cell transplant is the standard of care for fit patients with multiple myeloma (MM). However, many patients experience inadequate stem cell harvest after myeloid growth factor-based mobilization or suboptimal response to induction therapy. In the setting of high disease burden or heavy pretreatment, combination chemotherapy regimens, sometimes with addition of novel agents, may offer successful mobilization and debulking and include VTD-PACE, DCEP, and V-DCEP. We sought to offer a mobilization regimen that could 1) overcome mobilization failure and 2) debulk aggressive or refractory disease. We formulated the novel regimen VRD-CEP to achieve tolerability similar to that of DCEP but improve efficacy by combining a second-generation IMiD with a PI.
Methods: We performed a retrospective, single-institution evaluation of fit MM patients with history of inadequate mobilization, inadequate response to induction, or aggressive disease characteristics. Patients received bortezomib 1 mg/m2 SC days 1, 4, 8, and 11; DCEP (dexamethasone, cyclophosphamide, etoposide, and cisplatin) in standard fashion days 4-7; and lenalidomide 25 mg daily days 4-7 and again upon achieving platelet > 50. Patients received filgrastim 10 mcg/kg SC beginning day 10.
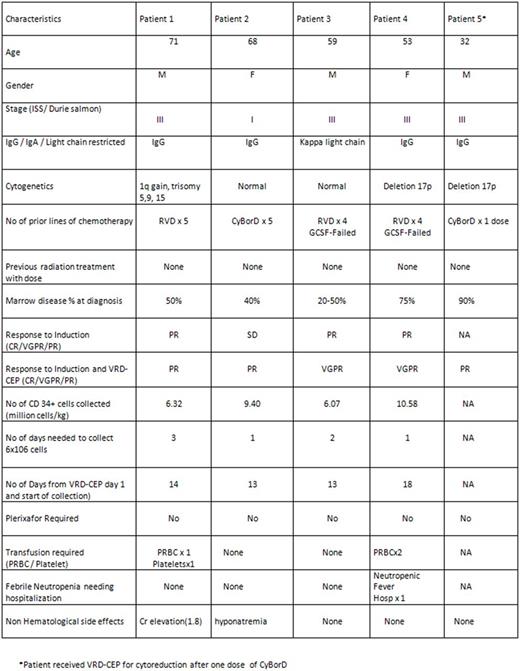
Results: Five patients were available for analysis; the median age was 59 (range 32-71). 2 patients were over 65, and 1 had ESRD. 2 patients underwent prior unsuccessful G-CSF + plerixafor mobilization, 2 patients had suboptimal response to induction, and 1 patient had aggressive disease features (plasmablastic histology, del 17p) with complete marrow replacement; 4 of 5 had ISS stage III disease. All patients received 1 cycle of the regimen. Three of the 4 mobilization patients achieved deepening of response based on M-protein or serum free light chains; one patient achieved near-complete elimination of plasma cells in the marrow and free light chain ratio normalization but could not undergo harvest due to logistical reasons. 3 of 5 patients were considered to have a PR or better. All patients treated with mobilization intent collected > 6 million CD34+ cells/kg (median 7.86; range 6.07-10.58 million/kg). The median time from the start of DCEP to apheresis was 13.5 days (range 13-18); the median number of apheresis days was 1.5 (range 1-3). No patients required dosing of plerixafor.
Toxicities were acceptable. Grade 4 thrombocytopenia occurred in 3 patients, grade 4 neutropenia occurred in 4, and PRBC transfusions were given to 2. 1 patient was hospitalized for neutropenic fever. 1 patient developed reversible grade 2 renal insufficiency, and 1 patient developed grade 3 hyponatremia attributed to cyclophosphamide. No patients experienced worsening neuropathy, syncope, hypotension, deep vein thrombosis, grade 3-4 nausea/vomiting, or diarrhea. Patient-specific details are listed in Table 1.
Discussion: Stem cell mobilization using G-CSF and plerixafor may be subject to inadequate yields in 1-40% of patients. Patients with significant residual plasma cell marrow involvement may require additional therapy to achieve adequate mobilization and cytoreduction prior to autologous transplant. VTD-PACE, DCEP, and V-DCEP have shown efficacy in relapsed or refractory MM patients. Such regimens are also useful for chemo-mobilization of stem cells. However, the efficacy of DCEP in the relapsed/refractory setting may be limited (40-50%), and VTD-PACE may have more limited applicability due to its higher toxicity burden. We believe that the addition of bortezomib and lenalidomide to DCEP achieves the desired potency to debulk refractory or high-risk disease while maximizing tolerability. More significantly, mobilization results were excellent, and we were able to achieve excellent stem cell yields in patients who had experienced inadequate growth factor mobilizations. The regimen merits further exploration in a larger cohort of patients.
Conclusion: In a preliminary cohort of 5 MM patients, VRD-CEP appears to be an effective and safe mobilization regimen in patients with residual marrow disease or those who failed prior mobilization attempts. VRD-CEP led to efficient cytoreduction in patients with high-risk cytogenetics and heavy burden of disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal